Chemistry: The fun science
Atomic Structure
What are atoms made of?
How will it affect its properties?
Previously...
You've learnt the behaviours of particles in their solid, liquid and gaseous states. But what are those particles made of?
A Brief History

Democritus
Around 460 B.C., a Greek philosopher Democritus asked this question:
If you break a piece of matter in half, then break it in half again, how many breaks will you have to make before it can break no further?
Democritus thought it must end at some point to get a smallest possible piece of matter that is not divisible. He called these basic matter particles, atoms.
Sadly, Aristotle (another prominent Greek philosopher) thought that was rubbish and people trusted him alot, so this line of inquiry was left aside until the 1800s, when John Dalton discovered that matter IS made of elementary lumpy particles - atoms!
Don't worry, bro, I got your back! *wink*

John Dalton
So what is an atom?
An atom is the smallest possible unit of a element that retains its chemical properties.
Let's Explore!
Just how small are atoms? Let's get a rough idea by watching the video below (skip from 3:00 to 3:37)
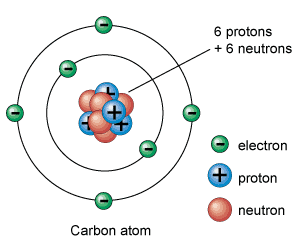
What are atoms made of?
An atom is made of three components:
-
Protons
-
Neutrons
-
Electrons
Game On!
Are you confident you are confident in differentiating protons, neutrons and electrons? Unlock all three grids!
Isotopes
Isotopes
Isotopes of the same element has the same number of protons, but different number of neutrons.
Remember that we sort atoms by their proton number, hence, even with a different mass number, they are still considered as being the same element!
Isotopes of the same element has the same number of protons, but different number of neutrons.
Remember that we sort atoms by their proton number, hence, even with a different mass number, they are still considered as being the same element!

Same same, but different!
Relative abundance
Because most elements have isotopes, their relative atomic mass is the average mass of the isotopes based on their relative abundance.
How can we determine the relative atomic mass of an elemnt? How can we determine the relative abundance of each isotope? Find out in this video!